Galvanized Rebar: It Works

Addressing corrosion after construction is expensive and disruptive as all or part of the structure needs to be closed periodically for maintenance and repair. Failure to properly address corrosion can also lead to catastrophic structural failures with potentially deadly consequences.
Many of these costs and safety issues are avoidable if the potential for corrosion is minimized during construction through proper design and material selection. This is especially critical for the reinforcing steel and the structural steel connectors used to strengthen and join concrete sections.
Galvanizing, the process where steel parts are immersed in a bath of molten zinc, has a proven track record and should be your first choice in corrosion protection—because it works.

There are two basic types of coatings: barrier and sacrificial. Most coatings can be classified as barrier because they provide basic protection from air and water penetration to the steel they are covering. Sacrificial or zinc coatings offer barrier protection, but also provide a secondary line of defense if the barrier coating is damaged as the zinc sacrifices itself, or corrodes preferentially before the steel.
All coating systems are designed to provide barrier protection to the substrate they cover. Barrier coatings are only effective as long as the coating remains intact. Any scratch, cut, or abrasion exposes the underlying steel to corrosive forces.
When steel protected only by a barrier coating is damaged or weathered, corrosion will initiate at the unprotected surface and quickly expand from that point outward. This is caused by the growth of iron oxides under the coating, which stresses the coating, causing failure and subsequent expansion of the unprotected area.
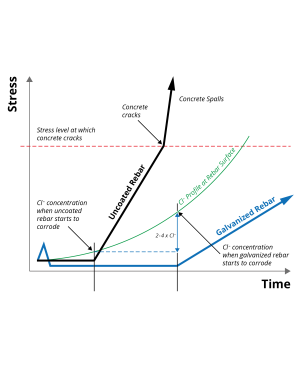
Galvanized zinc coatings form an impervious metallic zinc barrier around the steel to isolate the steel surface from the surrounding concrete. This barrier is the first line of defense in protecting the steel from corrosion.
- It offers excellent resistance to chloride salt attack and is unaffected by concrete carbonation
- Zinc’s cathodic protection inhibits corrosion at any minor coating discontinuity and also prevents ‘undercutting’ of the coating, confining any corrosion risk to the local area of exposed steel.
- Zinc corrosion results in little accompanying volume change. Unlike with steel corrosion, there is no adverse impact on the surrounding concrete. Research shows that any corrosion products simply diffuse into the adjacent concrete, helping to fill micro porosity that further inhibits corrosion
Galvanizing lengthens the life of steel and concrete structures enabling a huge conservation of natural resources by reducing the waste inherent with premature end-of-life.